UW Clinical Trials Center
The UW Clinical Trials Center (UW CTC) provides key leadership, infrastructure, and statistical project support to both complex and pragmatic single and multi-center clinical trials. Our expertise includes study design, conduct, analysis and reporting of results, trial management systems, regulatory knowledge, and overall project leadership.
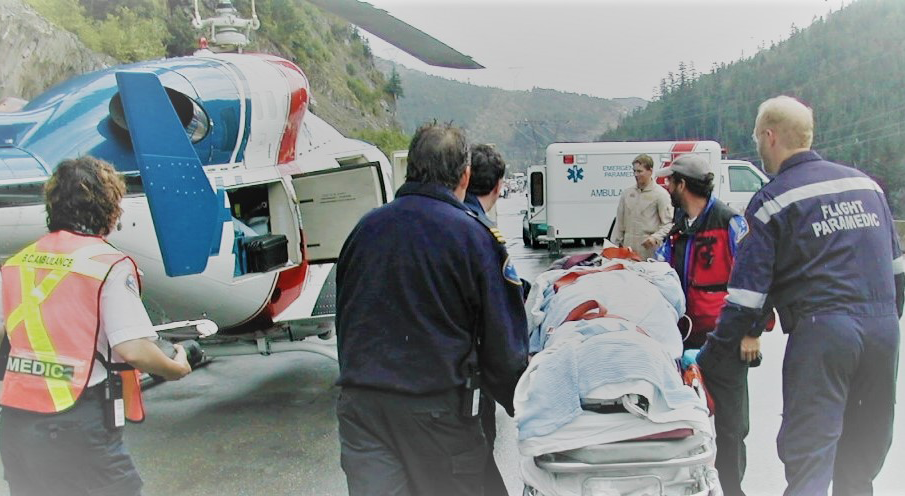
We have provided and are providing statistical support and/or support as the coordinating center e.g. for A-TREAT (NCT02578901) and SOUNDS trials (NCT05730283).
The UW Clinical Trials Center is an established team of biostatisticians, programmers, and project management staff that have extensive experience designing, planning, implementing, conducting, and monitoring small to large-scale clinical trials. They bring to the table a broad set of skills, and have an established foundation built on open communication and active collaborative problem solving that has ensured the success of many complex clinical trials.
The Center is a critical resource for investigators at the UW and nation-wide who are striving to improve clinical approaches in many different scientific research areas.
We welcome new opportunities for collaboration; please reach out to us at: uwctc@uw.edu
Current UW CTC studies include:
A-TREAT – This study compared the incidences of bleeding and thrombosis and the transfusion requirements in patients randomized to receive tranexamic acid (TXA) or a placebo for hypo-proliferative thrombocytopenia secondary to primary marrow disorders, chemotherapy, immunotherapy, radiation, and/or hematopoietic stem cell transplant.
SOUNDS – ORC-13661 is a new chemical entity being developed to prevent hearing loss in patients exposed to aminoglycoside antibiotics (AGs). AGs are an important class of broad spectrum antibiotics especially effective against gram-negative bacteria and Mycobacteria. Unfortunately, as many as 20% of patients administered parenteral AGs for over 5 days suffer permanent, measurable hearing loss, as do upwards of 60% of patients on long term therapy. Largely because of the ototoxic side effects, AGs are currently administered only for life threatening situations or where they are the only effective treatment. At this time there are no FDA approved drugs that have been shown to prevent any form of sensorineural hearing loss. Preventing AG-induced hearing loss would be of enormous benefit patients currently being treated with AGs. It would also allow this inexpensive and effective but underused class of antibiotics to be used more routinely. We are conductine a randomized, double-blind, placebo-controlled, multicenter, phase 2 study of ORC-13661 administered orally in conjunction with intravenously administered amikacin to patients with severe NTM infections. The goal of this to evaluate the efficacy of ORC-13661 for mitigation or prevention of ototoxicity.
FACT – The FACT Study will address fundamental treatment strategies involving first responder cardiopulmonary resuscitation of humans who suffer out-of-hospital cardiac arrest (SCA). Although fundamental to resuscitation, the optimal strategy for first responder CPR is derived largely from observational research. We are conducting a phase 2, factorial-design, cluster cross-over randomized controlled clinical trial among persons who suffer SCA to compare the survival effects of first responder treatment strategies related to airway management and chest compression rates during CPR. The study will enroll 4,200 subjects to compare the survival effects of first responder treatment strategies of bag valve mask (BVM) ventilation versus i-gel ventilation and chest compression rates of 100 versus 110 versus 120 per minute during CPR.
T.B. Gernsheimer, S.P. Brown, D.J. Triulzi, N.S. Key, N. El Kassar, H. Herren, J.N. Poston, M. Boyiadzis, B.N. Reeves, S. Selukar, M. Pagano, S.S. Emerson, S. May (joint senior author with S.S. Emerson) (2022) “Prophylactic tranexamic acid in patients with hematologic malignancy: a placebo controlled, randomized clinical trial”, Blood, Jun 7.
F. Kim, C. Maynard, C. Dezfulian, M. Sayre, P. Kudenhuk, T. Rea, D. Sampson, M. Olsufka, S. May, G. Nichol (2021) “Effect of Pre-hospital Sodium Nitrite on Survival to Hospital Admission in Out-of-Hospital Cardiac Arrest: A randomized clinical trial”, JAMA, 325(2), 138-145, doi:10.1001/jama.2020.24326.
Rowell SE, Meier EN, McKnight B, Kannas D, S May, Sheehan K, Bulger EM, Idris AH, Christenson J, Morrison LJ, Frascone RJ, Bosarge PL, Colella MR, Johannigman J, Cotton BA, Callum J, McMullan J, Dries DJ, Tibbs B, Richmond NJ, Weisfeldt ML, Tallon JM, Garrett JS, Zielinski MD, Aufderheide TP, Gandhi RR, Schlamp R, Robinson BRH, Jui J, Klein L, Rizoli S, Gamber M, Fleming M, Hwang J, Vincent LE, Williams C, Hendrickson A, Simonson R, Klotz P, Sopko G, Witham W, Ferrara M, Schreiber MA. (2020) “Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury.” JAMA. Sep 8;324(10):961-974. doi: 10.1001/jama.2020.8958. PubMed PMID: 32897344; PubMed Central PMCID: PMC7489866.
A.S. Ginsburg, T. Mvalo, E. Nkwopara, E.D. McCollum, M. Phiri, R. Schmicker, J. Hwang, C.B. Ndamala, A. Phiri, N. Lufesi, and S. May (2020) “Amoxicillin 3 vs 5 days for chest-indrawing pneumonia in Malawian children”, NEJM, 383, 13-23. DOI: 10.1056/NEJMoa1912400. PMID: 32609979
S. May, S.P. Brown, R.H. Schmicker, S. Emerson, E. Nkwopara and A.S. Ginsburg, (2019) “Non-inferiority designs comparing placebo to a proven therapy for childhood pneumonia in low resource settings”, Clinical Trials, Dec 8, DOI: 10.1177/1740774519888460. PMID: 31814441.
H.E. Wang, R.H. Schmicker, M.R. Daya, S.W. Stephens, A.H. Idris, J.N. Carlson, M.R. Colella, H. Herren, M. Hansen, N.J. Richmond, J.C.J. Puyana, T.P. Aufderheide, R.E. Gray, P.C. Gray, M. Verkest, P.C. Owens, A.M. Brienza, K.J. Sternig, S. May, G.R. Sopko, M.L. Weisfeldt, G. Nichol for the Resuscitation Outcomes Consortium Investigators, (2018) “Effect of a Strategy of Initial Laryngeal Tube Insertion vs. Endotracheal Intubation on 72-Hour Survival in Adults with Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial", JAMA 320(8):769-778. doi:10.1001/jama.2018.7044. PMID: 30167699.
A.S. Ginsburg, T. Mvalo, E. Nkwopara, E. McCollum, C.B. Ndamala, R. Schmicker, A. Phiri, N. Lufesi, R. Izadnegahdar, S. May (2018) “Placebo versus amoxicillin for non-severe fast breathing pneumonia in Malawian children aged two to 59 month: a double-blind, randomized controlled non-inferiority trial”, JAMA Pediatrics, doi:10.1001/jamapediatrics.2018.3407. PMID: 30419120.
Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, Christenson J, Egan D, Ornato JP, Weisfeldt ML, Stiell IG, Idris AH, Aufderheide TP, Dunford JV, Colella MR, Vilke GM, Brienza AM, Desvigne-Nickens P, Gray PC, Gray R, Seals N, Straight R, Dorian P, and the ROC Investigators. New England Journal of Medicine 2016; 374:1711-1722 Link
Trial of continuous or interrupted chest compressions during CPR. Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S, Christenson J, Kudenchuk P, Vaillancourt C, Rea TD, Idris AH, Colella R, Isaacs M, Straight R, Stephens S, Richardson J, Condle J, Schmicker RH, Egan D, May S, Ornato JP, and the ROC Investigators. New England Journal of Medicine 2015; 373(23):2203-2214 Link
Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs. a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR Randomized Clinical Trial. Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O’Keeffe T, Rizoli S, Robinson BRH, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet J-F, Hoyt DB, Pearson GD, Leroux B, van Belle G for the PROPPR Study Group. JAMA 2015; 313(5):471-482. PMCID: PMC4374744 Link
The impact of peri-shock pause of survival from out-of-hospital shockable cardiac arrest during the Resuscitation Outcomes Consortium PRIMED trial. Cheskes S, Schmicker RG, Verbeek PR, Salvido DD, Brown SP, Brooks S, Menegazzi JJ, Vaillancourt C, Powell J, May S, Berg RA, Sell R, Idris A, Kampp M, Schmidt T, Christenson J, and the ROC investigators. Resuscitation 2014; 85:336-342. PMCID: PMC3944081 Link
Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. Stiell IG, Nichol G, Leroux BG, Rea TD, Ornato JP, Powell J, Christenson J, Callaway CW, Kudenchuk PJ, Aufderheide TP, Idris AH, Daya M, Wang HE, Morrison L, Davis D, Andrusiek D, Stephens S, Cheskes S, Schmicker RH, Fowler R, Vaillancourt C, Hostler D, Zive D, Pirrallo RG, Vilke GM, Sopko G, Weisfeldt M, and the ROC Investigators. New England Journal of Medicine 2011; 365(9):787-797. PMCID: PMC3181067 Link
A trial of an impedance threshold device in out-of-hospital cardiac arrest. Aufderheide TP, Nichol G, Rea TD, Brown SP, Leroux BG, Pepe PE, Kudenchuk PJ, Christenson J, Daya MR, Dorian P, Callaway CW, Idris AH, Andrusiek D, Stephens SW, Hostler D, Davis DP, Dunford JV, Pirrallo RG, Stiell IG, Clement CM, Craig A, Van Ottingham L, Schmidt TA, Wang HE, Weisfeldt ML, Ornato JP, Sopko G, ROC Investigators. New England Journal of Medicine 2011; 365:798-806. PMCID: PMC3204381 Link
Perishock Pause: an independent predictor of survival from out-of-hospital shockable cardiac arrest. Cheskes S, Schmicker RH, Christenson J, Salcido DD, Rea T, Powell J, Edelson DP, Sell R, May S, Menegazzi JJ, Van Ottingham L, Olsufka M, Pennington S, Simonini J, Berg RA, Stiell I, Idris A, Bigham B, Morrison L, on behalf of the Resuscitation Outcomes Consortium (ROC) Investigators. Circulation 2011; 124:158-166. PMCID: PMC3138806 Link
Out-of-hospital hypertonic resuscitation following traumatic hypovolemic shock: a randomized, placebo controlled trial. Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, Brasel KJ, Tisherman SA, Coimbra R, Rizoli S, Minei JP, Hata JS, Sopko G, Evans DC, Hoyt DB, ROC Investigators. Annals of Surgery 2011; 253(3):431-441. PMCID: PMC3015143 Link
Ventricular tachyarrythmias following cardiac arrest in public versus home. Weisfeldt ML, Everson-Stewart S, Sitlani C, Rea T, Aufderheide TP, Atkins DL, Bigham B, Brooks SC, Foerster C, Gray R, Ornato JP, Powell J, Kudenchuk PJ, Morrison LJ, ROC Investigators. New England Journal of Medicine 2011; 364:313-21. PMCID: PMC3062845 Link
Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. Bulger EM, May S, Brasel KJ, Schreiber M, Kerby JD, Tisherman SA, Newgard C, Slutsky A, Coimbra R, Emerson S, Minei JP, Bardarson B, Kudenchuk P, Baker A, Christenson J, Idris A, Davis D, Fabian TC, Aufderheide TP, Callaway C, Williams C, Banek J, Vaillancourt C, van Heest R, Sopko G, Hata JS, Hoyt DB, ROC Investigators. Journal of the American Medical Association 2010; 304(13):1455-1464. PMCID: 3015143 Link
Receiving hospital characteristics associated with survival after out-of-hospital cardiac arrest. Callaway C, Schmicker R, Kampmeyer M, Powell J., Rea T, Daya M, Aufderheide T, Davis D, Rittenberger J, Idris A, Nichol G, ROC Investigators. Resuscitation 2010; 81(6) 524-529. PMCID: PMC2856722 Link
Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The ROC epistry-cardiac arrest. Atkins D, Everson-Stewart S, Sears G, Daya M, Osmond M, Warden C, Berg R. Circulation 2009; 119(11):1484-1491. PMCID: PMC2679169 Link
Regional variation in out-of-hospital cardiac arrest incidence and outcome. Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell IG. Journal of the American Medical Association 2008; 300:1423-1431. PMCID: PMC3187919 Link
Phone: (206) 685-1302
Email: uwctc@uw.edu